By Karen Knapstein
When electrochemically dissimilar metals are in contact with one another, galvanic action occurs. The direct contact creates a conductive path for electrons and ions to move from one metal to the other; the result is accelerated corrosion.
For example, consider the Statue of Liberty. Lady Liberty has a copper surface on a cast iron frame. The two metals were originally separated by an insulating material. When that insulating material failed, the result was a great deal of galvanic corrosion.
Three conditions must exist for galvanic corrosion to occur:
1 There must be two electrochemically dissimilar metals present.
2 There must be an electrically conductive path between the two metals.
3 There must be a conductive path for the metal ions to move from the more anodic metal to the more cathodic metal.
If any one of these three conditions doesn’t exist, galvanic action won’t take place.

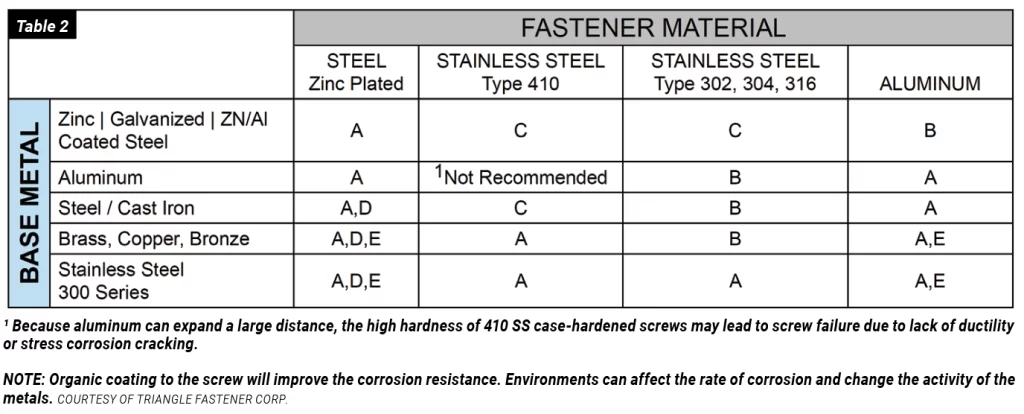
Galvanic corrosion should be a concern in the use of metal fasteners such as bolts, screws, and welds. According to an article in Preservation Science, “Because fasteners have a much smaller surface area than the materials they fasten, fasteners that take on the role of the anode will be at risk of rapid corrosion and thus should be avoided. For example, zinc-coated fasteners should only be used to connect steel coated with aluminum, zinc, and Galvalume, as these are very close on the Galvanic Series and are not generally at risk of corrosion when placed together. On the other hand, zinc-coated or aluminum-coated fasteners should not be used to attach copper or stainless-steel panels.”
Andrew Mullen, President of Direct Metals, Inc., advised, “It is important to understand the differences between metal alloys and how bare dissimilar metals in certain environmental conditions can have serious reactions that promote premature corrosion and degradation.”
To minimize the risk of galvanic corrosion occurring in fasteners, the surface metal on the fastener should be matched with the surface metal it will fasten. The most desired combination is to have a large anode with a small cathode; in other words, fasteners such as bolts and screws should be made of the metal less likely to corrode, or the more cathodic.
If it’s not possible to avoid using dissimilar metals, coatings play a critical role in eliminating the risk of galvanic action. A non-conductive coating acts as a barrier, removing the connection between them. Common coating practices that prevent galvanic corrosion include, but are not limited to, zinc plating, galvanizing, and powder coating.
In the galvanic table shown above (Table 1), the closer the metals are to one another on the list, the less likely they are to react to one another and experience galvanic corrosion.
John Sheridan, owner of Sheridan Metal Resources, teaches as part of his training course: “Aluminum has a similar protective coating as zinc, eliminating risk of corrosion. Galvanized steel is coated with a fine film of zinc, so this zinc-zinc contact poses no threats. Copper and zinc don’t play nicely together. Copper runoff will stain the zinc. Steel that is non-galvanized should also be avoided, as the similar electron transfers between the metals will result in corrosion and deterioration. In addition, zinc is not compatible with oak, chestnut, red or white cedar, Douglas fir, and any woods with a pH less than 5.”
There is a good chance you already knew from experience that some fasteners react badly to certain materials. Now you know why. Remember: Your supplier will be happy to help you select the appropriate fastener for whatever materials you’re working with.

Article Corrections and Clarifications
After this article was published, we received some additional information that more accurately explains galvanic action. The following corrections and clarifications were offered by Rob Haddock representing the Metal Roof Advisory Group, Metal Construction Association.
Noted Haddock:
“This article has some good information, but in total is a bit misleading due to not expounding the complete story.
It leaves one to rely 100% on the galvanic scale as the beginning, middle and end of the story. It isn’t. It may be the beginning, but far from the end in many common applications. The bottom line is that while this scale is the first thing most people grab to rely upon as a technical resource– it does not tell the whole truth and therefore can be very misleading.
“The galvanic scale gives the order of electro-chemical behavior and therefore compatibility– but of the parent (or base) metals only. It does not consider metal oxide layers, and because all metals form oxides– well, that’s the rest of the story. The oxide is a different material than the parent metal that created it and often behaves as an insulator, preventing (or retarding) electron exchange (galvanic action) and NOT necessarily reflecting galvanic compatibility of the parent metals.
“The galvanic scale reflects electrolytic behavior solely of the parent metal, so it only tells the whole truth when oxide layers are not involved- and that only happens when the electrolyte is very aggressive (acetic -e.g. sea water) For these reasons, it should not be relied upon as a sole information source. In fact, one could say that it only tells the complete truth when in the presence of salt spray or other chlorides.
“By way of example: Aluminum forms a durable oxide very rapidly in the presence of air and humidity. This is also sometimes induced by a chemical process (anodization), but it also happens naturally just with exposure. Aluminum oxide is a barrier material coating the aluminum– and it is electrically non-conductive, so electrons are impeded from passage through it from anode-to-cathode. One can use anodized or just aluminum that has oxides formed on a bare copper roof without incident in most environments. Those two metals are very distant on the scale, but compatible owing to the aluminum oxide layers.
“I also read in there that dissimilar metals in direct contact always result in corrosion. Not true. Moisture is needed to establish an electrolyte. If the connection is kept dry, there is no electrolytic contact and no corrosive effect. Understanding corrosive behaviors of metals requires a much deeper dive than the galvanic scale because various metal oxides in varying environments all behave differently.
“Incidentally, the Metal Construction Association publishes a Fastener Compatibility Guide that takes into consideration oxide layers and also longevity/durability considerations. It is a metal roofing industry consensus document (as opposed to what one single company or another has to say on the subject) and therefore one of the best practical resources out there.”
Rob Haddock
The Metal Roof Advisory Group